Ovarian cancer remains a major medical problem throughout the world. A striking example is high-grade serous ovarian cancer (HGSC), the most common and lethal of all epithelial ovarian carcinomas with a five-year survival rate of less than 40% [1]. HGSC was recently found to originate not from the ovarian surface, as previously believed, but from secretory cells of the distal oviduct (Fallopian tube) [2, 3]. However, we have little understanding of these secretory cells or their normal roles in signaling to the ovary.
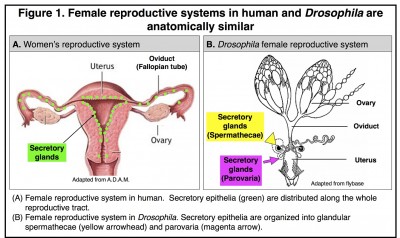
Similar secretory cells also exist in the female reproductive tract of Drosophila melanogaster, whose reproductive system is anatomically similar to human (Fig 1).
These secretory cells are organized into two types of glands named spermathecae and parovaria and are essential for reproductive success as are the secretory cells in mammals. We recently defined the secretory cell lineage at the single cell resolution and discovered that gland formation utilizes a conserved genetic program including the Notch signaling pathway, NR5A family member Hr39 (homologous to Lrh-1 in mammals), and Runt-domain transcription factor Lozenge [4] (See the video below). By manipulating secretory cell number or their adult function, we further discovered that secretions from these glands play conserved roles in regulating ovulation (egg release from the ovary) and sperm function [5]. It is intriguing that Lrh-1 is also required in mammals for ovulation and that the number of ovulation cycles is positively correlated with the risk of ovarian cancer[6, 7]. Therefore, Drosophila becomes a valuable model for understanding the basic biology of oviduct secretory cells and their contribution to cancer formation [8].
Currently, the major focuses in the Sun lab are:
- Dissecting the molecular mechanism for secretory cell differentiation;
- Identifying the secreted products and molecular mechanisms controlling ovulation and sperm function.
With the powerful genetic tools available in Drosophila, we have identified multiple candidate genes required for secretory cells differentiation and for regulating ovulation and sperm function. We will further characterize the function of these genes and their regulation. We will then determine the function of their homologous genes in mice. Using this comparative approach, we hope to understand the conserved pathways regulating oviduct secretory cell formation and physiological function in humans, to provide the basis for understanding the origin of ovarian cancer, and to develop biomarkers for early diagnosis of ovarian cancer.
- Vaughan, S., Coward, J.I., Bast, R.C., Jr., Berchuck, A., Berek, J.S., Brenton, J.D., Coukos, G., Crum, C.C., Drapkin, R., Etemadmoghadam, D., et al. (2011). Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer 11, 719-725.
- Levanon, K., Crum, C., and Drapkin, R. (2008). New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol 26, 5284-5293.
- Berns, E.M., and Bowtell, D.D. (2012). The changing view of high-grade serous ovarian cancer. Cancer Res 72, 2701-2704.
- Sun, J., and Spradling, A.C. (2012). NR5A Nuclear Receptor Hr39 Controls Three-Cell Secretory Unit Formation in Drosophila Female Reproductive Glands. Curr Biol 22, 862-871.
- Sun, J., and Spradling, A.C. (2013). Ovulation in Drosophila is controlled by secretory cells of the female reproductive tract. Elife 2, e00415.DOI: http://dx.doi.org/10.7554/eLife.00415
- Purdie, D.M., Bain, C.J., Siskind, V., Webb, P.M., and Green, A.C. (2003). Ovulation and risk of epithelial ovarian cancer. Int J Cancer 104, 228-232.
- Duggavathi, R., Volle, D.H., Mataki, C., Antal, M.C., Messaddeq, N., Auwerx, J., Murphy, B.D., and Schoonjans, K. (2008). Liver receptor homolog 1 is essential for ovulation. Genes Dev 22, 1871-1876.
- VijayRaghavan, K., and Rath, S. (2013). Evolution, ovulation and cancer. Elife 2.DOI: http://dx.doi.org/10.7554/eLife.00729